Our partners











Engineering for success
Thanks to this patented technology, we are pioneers in the design and production of human Fc monoclonal antibodies.

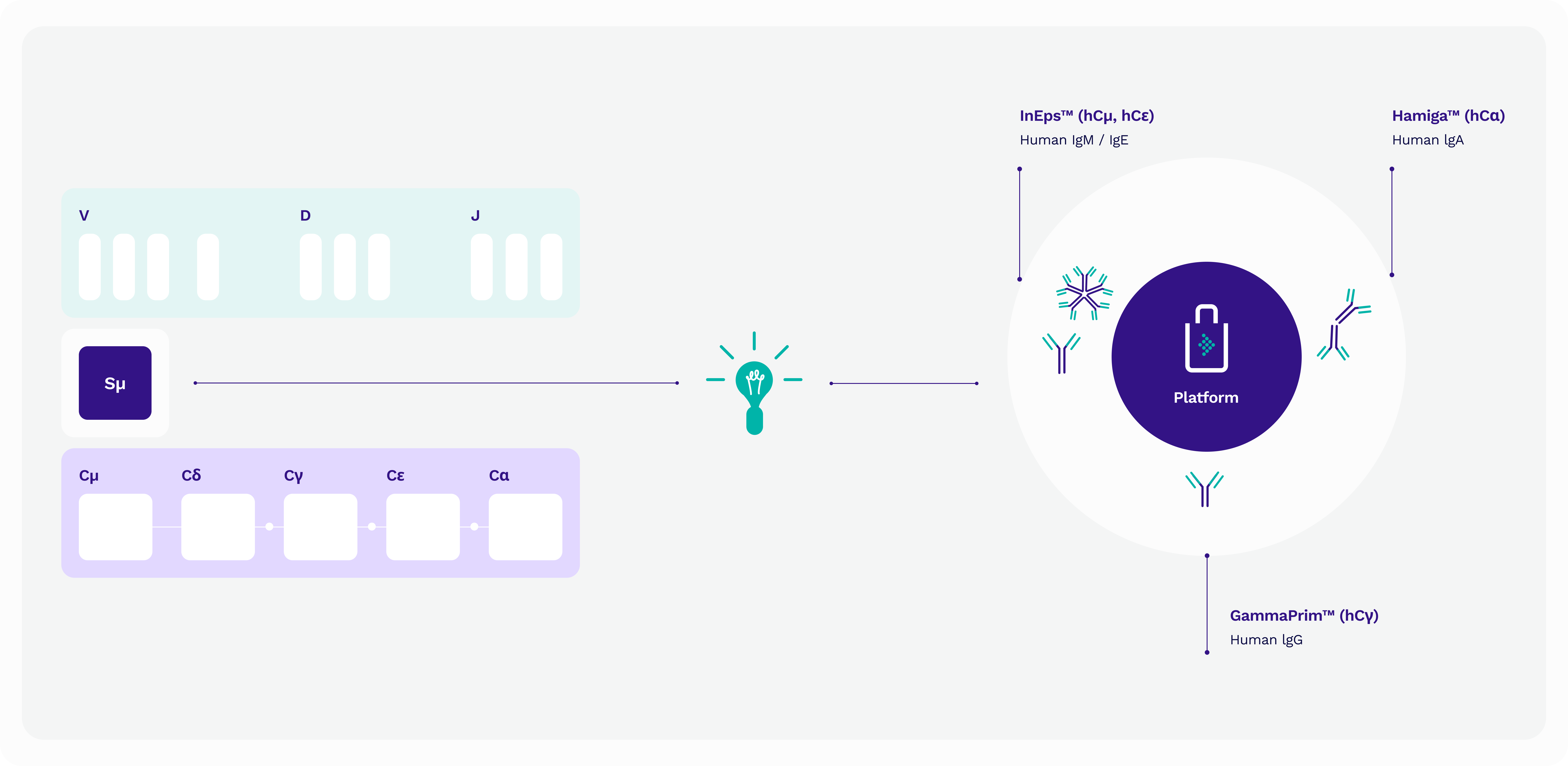
Our patented technology platform
With our unique patented technology platform, each mouse line hosts only one human isotype, ensuring unmatched specificity and consistency in antibody production. Coupled with fully human Fc regions, our antibodies are setting a new standard for precision diagnostics. Moreover, our platform preserves the complete immune repertoire of the mouse, allowing for unparalleled exploration of antigen-antibody interactions. By maintaining the natural VDJ recombination and hypermutation pathway, we uphold the highest quality standards, delivering reliable and reproducible results every time.
Patents
Immunoglobulin A antibodies
Transgenic non-human mammal for producing chimeric human immunoglobulin A antibodies
Immunoglobulin G antibodies
Transgenic non-human mammal for producing chimeric human immunoglobulin G antibodies
Immunoglobulin E antibodies
Transgenic non-human mammal for producing chimeric human immunoglobulin E antibodies

