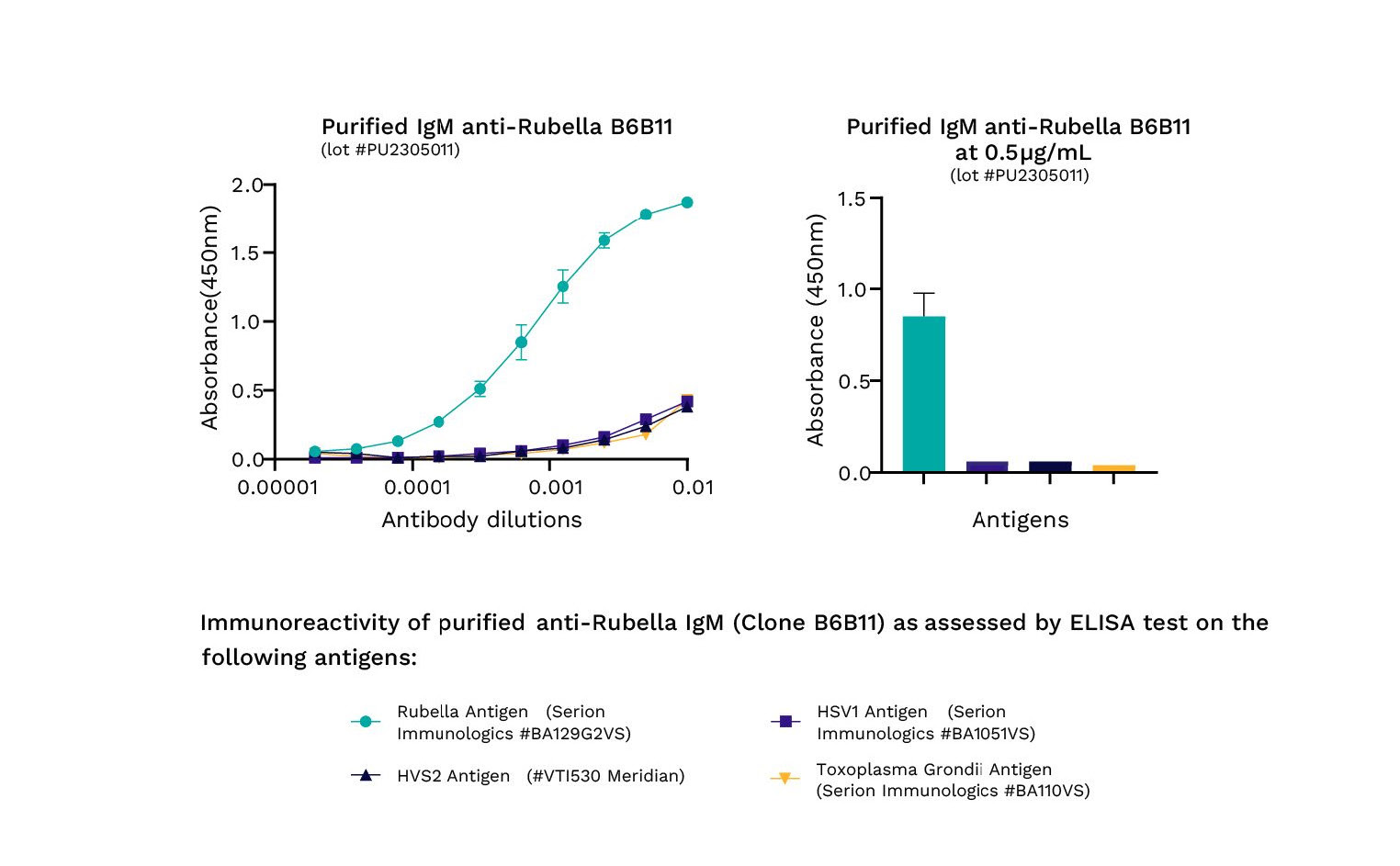
Our approach extends beyond IVD applications
Our human Fc monoclonal antibodies (mAbs) have significant potential for both research and industrial applications. We partnered with Professors Gruel, Rollin, and Pouplard, thrombosis experts from the University of Tours, France, to develop a human Fc mAb for studying heparin-induced thrombosis (HIT).
Our human Fc mAbs
We collaborated with with Prof. Gruel, Rollin, and Pouplard, a world-renowned team of thrombosis researchers, from the University of Tours, France, on a groundbreaking project: a human Fc monoclonal antibody (mAb) to study heparin-induced thrombosis (HIT).
- Research: The several clones developed for this project mimic the different antibodies that are found in patients.
- VD: 1 product for assay calibration, 1 cell assay, 1 external quality control
- Therapeutic: 1 mAb candidate for ADC
Advantages of IVD human Fc monoclonal antibodies
By incorporating our human Fc monoclonal mAbs into your IVD kit development and quality control processes, you gain access to a powerful tool for ensuring assay accuracy, reliability, and regulatory compliance.
Frequently asked questions and answers
Ready to switch? Here's how
Leading specialists in producing premium mAbs

Bcell Design is a leading French Life Science company specializing in the development and production of premium human Fc monoclonal antibodies (mAbs). Using our advanced knowledge and patented technology, we provide innovative solutions for diagnostics, research, and therapeutics.
Our mission is to provide scientists with reliable and effective human Fc monoclonal antibodies, accelerating the development of groundbreaking diagnostics, treatments, and therapies.
Our vision is to revolutionize healthcare by developing and producing accessible and transformative human Fc monoclonal antibodies, empowering scientists by improving the efficiency and reliability of diagnostics and unlocking cures for a healthier tomorrow.
Our company values
Long-term stewardship
Embracing progress
Uncompromising quality
Customer-centric innovation
Benefits of our custom monoclonal antibody development services

Unmatched specificity for every application
our mAbs target only the molecule you care about, minimizing unwanted interactions and ensuring the accuracy of your diagnostic tests (IVD).

Enhanced assay sensitivity
our custom monoclonal antibody development services enable us to engineer mAbs with superior sensitivity, allowing for the detection of even low levels of target analytes, crucial for early disease diagnosis and monitoring.

Scalable manufacturing
Our custom monoclonal antibody development process comes with large scale production capabilities to meet your needs.

Expert guidance every step of the way
our team of scientists is here to support you. We’ll help you select the perfect antigen, optimize the development process, and overcome any hurdles that arise. Together, we’ll create the mAb that perfectly aligns with your goals.
Heading
Sub- Heading
Fermentum eu commodo in diam. Tortor auctor sit imperdiet massa in egestas arcu tempus. Donec nisi ornare sit id pellentesque. Tincidunt id ultrices convallis nullam ante pulvinar facilisis bibendum egestas.
Non convallis massa placerat enim porta congue augue euismod. Ante in orci netus convallis vel mattis pellentesque porttitor. Volutpat vitae magna neque nec praesent pellentesque suspendisse vulputate volutpat. At commodo sit
Metus vulputate elit pulvinar eu elit viverra massa. Habitant scelerisque eget dui ipsum pharetra dolor. Nec at dapibus ac sit tellus ornare tincidunt.
E in egestas arcu tempus. Donec nisi ornare sit id pellentesque. Tincidunt id ultrices convallis nullam ante pulvinar facilisis bibendum egestas. Metus vulputate elit pulvinar eu elit viverra massa.
Habitant scelerisque eget dui ipsum pharetra dolor. Nec at dapibus ac sit tellus ornare tincidunt. Id erat nibh quam semper pellentesque sodales sit fusce. Facilisis sed velit eget pharetra pellentesque. Enim nulla aliquam volutpat sed a in euismod arcu. Ornare et at eget nibh cras lobortis sem.Lorem ipsum dolor sit amet consectetur. Purus egestas eget varius nunc. Dolor molestie eu fringilla imperdiet. Proin mauris

ISO certification
Bcell Design facility is certified under International Organization for Standardization (ISO) 9001:2015. The ISO 9001:2015 certification specifies globally recognized requirements for quality systems, feedback data management, and controlled manufacturing processes to ensure safe, reliable products

ISO certification
Bcell Design facility is certified under International Organization for Standardization (ISO) 9001:2015. The ISO 9001:2015 certification specifies globally recognized requirements for quality systems, feedback data management, and controlled manufacturing processes to ensure safe, reliable products

ISO certification
Bcell Design facility is certified under International Organization for Standardization (ISO) 9001:2015. The ISO 9001:2015 certification specifies globally recognized requirements for quality systems, feedback data management, and controlled manufacturing processes to ensure safe, reliable products
Lorem ipsum dolor sit amet consectetur. Purus egestas eget varius nunc. Dolor molestie eu fringilla Non convallis massa placerat enim ta congue augue euismod. Ante in orci netus convallis vel mattis ntesque porttitor. Volutpat vitae magna neque nec praesent lentesque suspendisse vulputate volutpat.
Our management team
Reviews from client and partners
How do our mAbs development services work?
Our robust custom monoclonal antibody development process empowers you to generate mAbs with a human Fc domain for IVD. This meticulous approach ensures the efficient generation of high-affinity, specificity and selectivity tailored mAbs to suit your specific needs
Custom made development process
Bcell Design is a leading French Life Science company specializing in the development and production of premium human Fc monoclonal antibodies (mAbs). Using our advanced knowledge and patented technology, we provide innovative solutions for diagnostics, research, and therapeutics.